Chemistry Lewis Structure
Chemistry Lewis Structure
Qno 3: Lewis structure nof
Ans: In the Nof Lewis structure, Nitrogen (N) is the least electronegative atom and is the center of the whole lewis structure. In the lewis structure there are totally 18 valence electrons.

Qno 4: Lewis structure for nof
Ans:
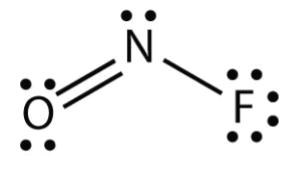
Q no 5: nof lewis structure
Ans: To determine the lewis structure we take the valence electrons of each atom. In this case, Nitrogen has 5 valence electrons, Oxygen has 6 and Fluorine has 7 and we get 18 when we add them up. Since Nitrogen is the center of the structure, we now have to place Oxygen and Fluorine around the Nitrogen.

Q no 16: n3 electron configuration
Ans: The electron configuration of N3- is N3-: 1s22s22p6 .
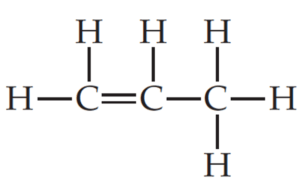
Q no 27: lewis structure of ch2ch3ch3
Ans:
Q no 28: Sbr4 lewis structure
Ans: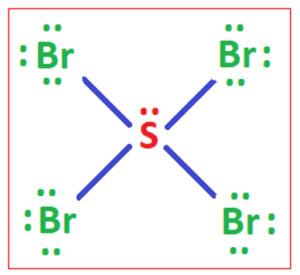
Q no 33: draw the main lewis structure of nof
Ans:
Q no 37: select the properties of the sn2 reaction mechanism
- Stereospecific- 100% inversion of configuration at the reaction site
- Bi-molecular at rate determining step
- Second order
- Rate is governed by steric effects.
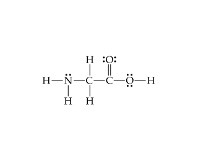
Q no 40: Lewis structure glycerine
Q no 57: Mass of a pencil
Ans: A standard unsharpened pencil with an unused eraser would weigh approximately 6 to 7 grams.